Sn Dopants with Synergistic Oxygen Vacancies Boost CO2 Electroreduction on CuO Nanosheets to CO at Low Overpotential
Sn Dopants with Synergistic Oxygen Vacancies Boost CO2 Electroreduction on CuO Nanosheets to CO at Low Overpotential
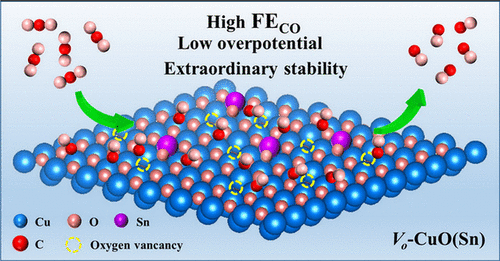
Using the electrochemical CO2 reduction reaction (CO2RR) with Cu-based electrocatalysts to achieve carbon-neutral cycles remains a significant challenge because of its low selectivity and poor stability. Modulating the surface electron distribution by defects engineering or doping can effectively improve CO2RR performance. Herein, we synthesize the electrocatalyst of Vo-CuO(Sn) nanosheets containing oxygen vacancies and Sn dopants for application in CO2RR-to-CO. Density functional theory calculations confirm that the incorporation of oxygen vacancies and Sn atoms substantially reduces the energy barrier for *COOH and *CO intermediate formation, which results in the high efficiency, low overpotential, and superior stability of the CO2RR to CO conversion. This electrocatalyst possesses a high Faraday efficiency (FE) of 99.9% for CO at a low overpotential of 420 mV and a partial current density of up to 35.22 mA cm–2 at −1.03 V versus reversible hydrogen electrode (RHE). The FECO of Vo-CuO(Sn) could retain over 95% within a wide potential area from −0.48 to −0.93 V versus RHE. Moreover, we obtain long-term stability for more than 180 h with only a slight decay in its activity. Therefore, this work provides an effective route for designing environmentally friendly electrocatalysts to improve the selectivity and stability of the CO2RR to CO conversion.