High-rate and selective conversion of CO2 from aqueous solutions to hydrocarbons
High-rate and selective conversion of CO2 from aqueous solutions to hydrocarbons
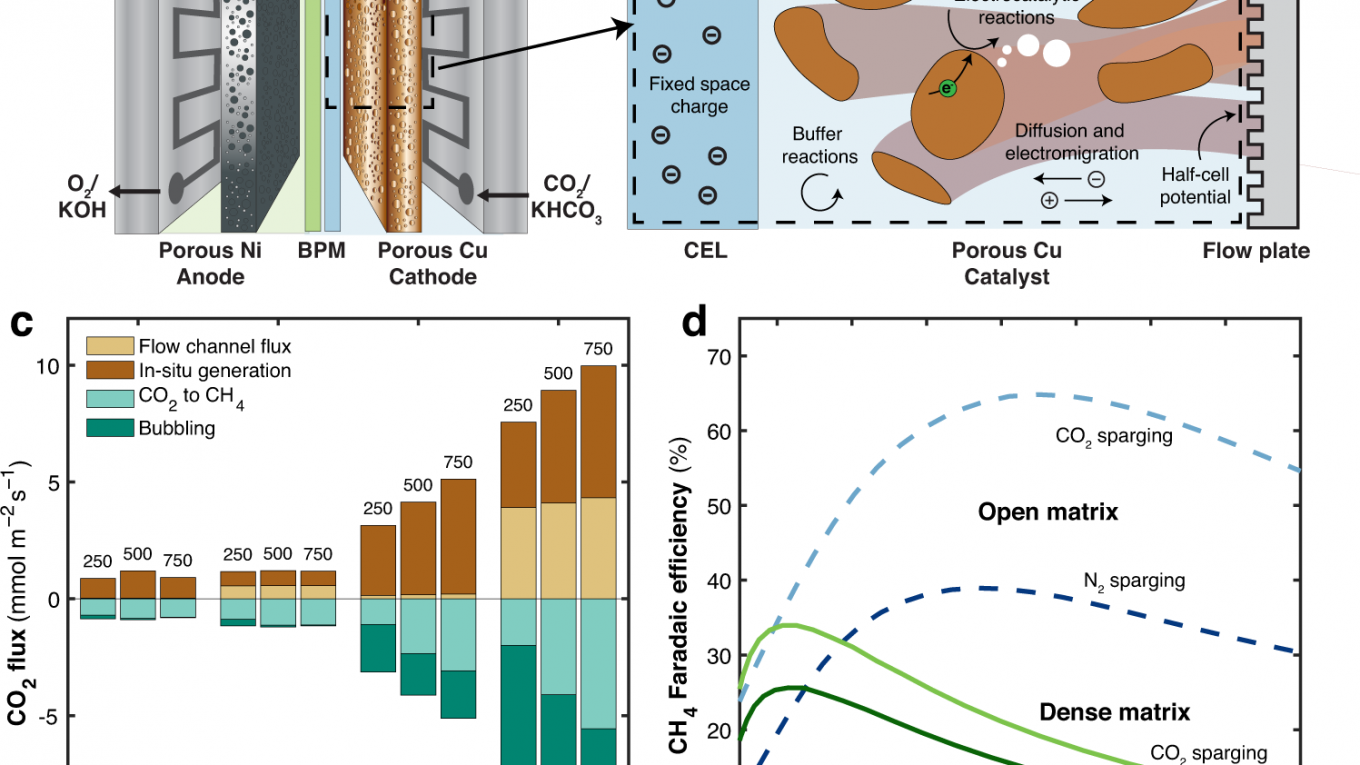
Electrochemical carbon dioxide (CO2) conversion to hydrocarbon fuels, such as methane(CH4), offers a promising solution for the long-term and large-scale storage of renewable electricity. To enable this technology, CO2-to-CH4 conversion must achieve high selectivity and energy efficiency at high currents. Here, we report an electrochemical conversion system that features proton bicarbonate-CO2 mass transport management coupled with an in-situ copper (Cu) activation strategy to achieve high CH4 selectivity at high currents. We find that open matrix Cu electrodes sustain sufficient local CO2 concentration by combining both dissolved CO2 and in-situ generated CO2 from the bicarbonate. In-situ Cu activation through alternating current operation renders and maintains the catalyst highly selective towards CH4. The combination of these strategies leads to CH4 Faradaic efficiencies of over 70% in a wide current density range (100 – 750 mA cm-2 ) that is stable for at least 12 h at a current density of 500 mA cm-2. The system also delivers a CH4 concentration of 23.5% in the gas product stream.