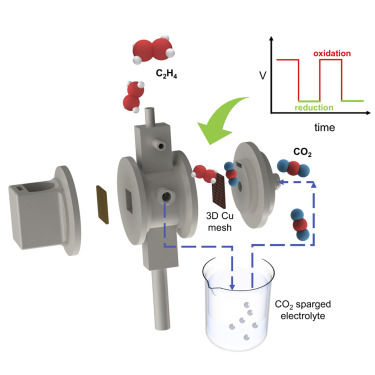
CO2-free high-purity ethylene from electroreduction of CO2 with 4% solar-to-ethylene and 10% solar-to-carbon efficiencies
Summary
C2H4 is an essential precursor for synthesis of a range of industrial chemicals while contributing ∼150 Mt of CO2e emissions per year. C2H4 synthesis via electrochemical CO2 reduction reaction (CO2RR) is an attractive approach to reduce carbon emissions. The lower single-pass conversion (<10%) of the state-of-the-art CO2 electrolyzers contributes significantly to the cost of post-CO2RR separation of products, rendering even processes with high CO2RR current densities unfit for scaling up. Here, we develop an aqueous flow-through electrochemical cell to enhance the activity and selectivity of C2H4 on a three-dimensional (3D) Cu mesh electrode by applying square-wave oscillating potentials. A high C2H4 faradaic efficiency of ∼58%, C2H4 current density of 306 mA/cm2, and gaseous C2H4 purity of ∼52 wt % without CO2 in the product stream are obtained. Integrating the 3D Cu mesh catalyst in a photovoltaic (PV) electrolyzer yields a solar-to-carbon (STC) efficiency of ∼10% with a solar-to-C2H4 efficiency of ∼4%.